Lipid Replacement Therapy (LRT): Rebuilding Cell Membranes From the Ground Up
The Structural Foundation Behind Cellular Energy, Signaling, and Brain Health
In APOE4 Revealing Its Hand, I focused on how APOE4 alters lipid transport and metabolism; here, I want to step back and examine something even more fundamental - the lipid architecture that makes neural signaling, repair, and resilience possible.
That structure is lipids.
This topic has become central in my own efforts to silence the expression of my APOE4/4 genes, not by chasing single targets, but by supporting the structural foundation that everything else depends on.
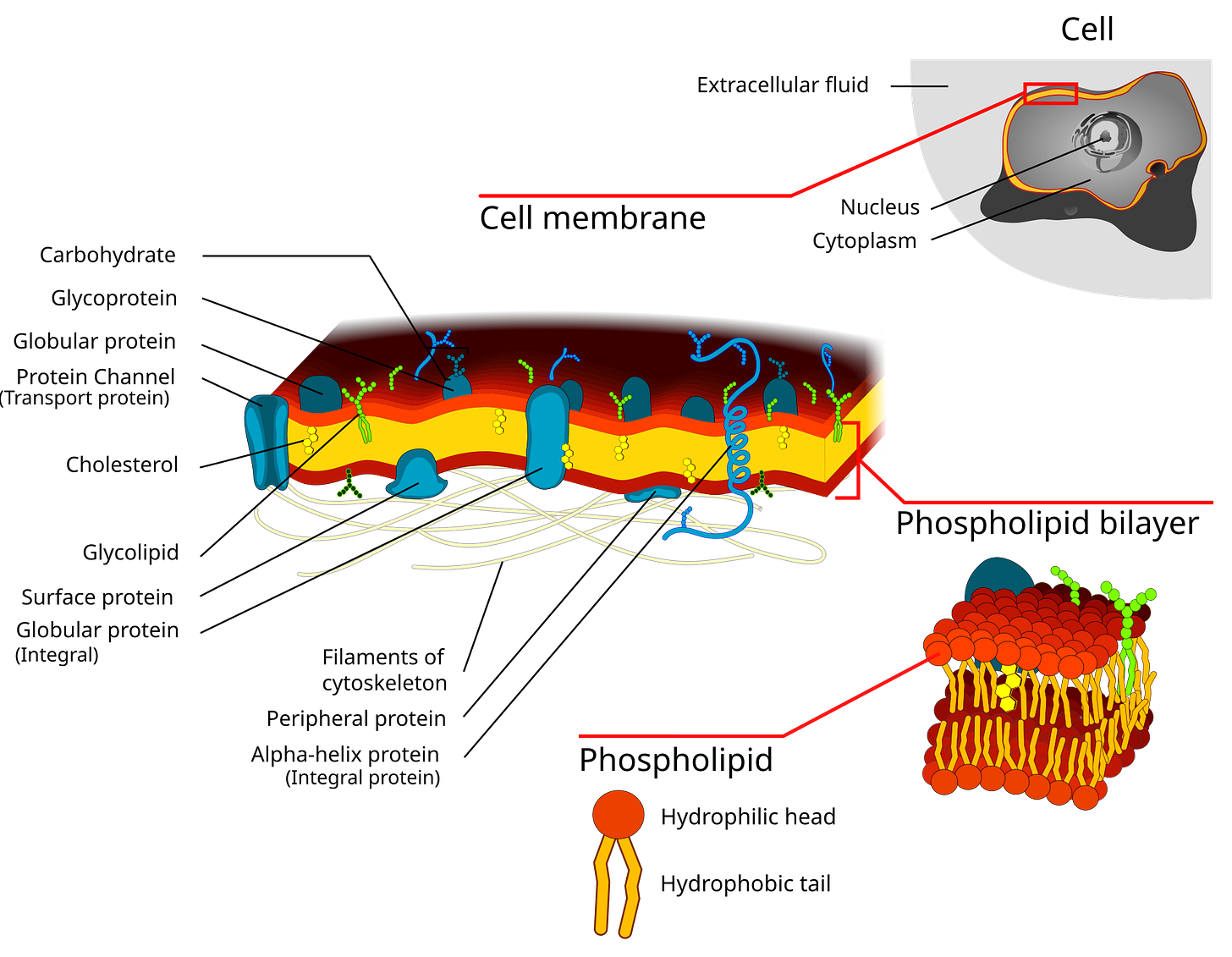
Why Lipids Matter More Than They Get Credit For
Every cell in the body is enclosed by a phospholipid bilayer. Inside each cell, mitochondria - our energy engines - are also wrapped in highly specialized lipid membranes.
These membranes determine:
how nutrients enter the cell
how waste products leave
how receptors respond to hormones and neurotransmitters
how efficiently mitochondria produce ATP
how much oxidative stress and inflammation accumulate
When membrane lipids are damaged, depleted, or replaced with inferior substitutes, cellular function degrades - even when standard lab values appear “normal.”
This is especially relevant for those of us with APOE4, a genotype known for inefficient cholesterol efflux. That reality naturally leads to a deeper question: what is actually controlling cholesterol movement at the cellular level?
Cholesterol Doesn’t Simply “Leave” a Cell
Cells don’t dump cholesterol into the bloodstream on their own.
Cholesterol exits cells through active export systems embedded in the cell membrane, primarily transporters such as ABCA1 and ABCG1. These transporters:
sit within the lipid bilayer
require a fluid, well-composed membrane to function
load cholesterol onto lipid-poor particles such as HDL or ApoE
So cholesterol efflux isn’t just about how much cholesterol is inside a cell -
it’s also about the condition of the membrane it must pass through.
ABCA1: A Quiet but Critical Player in Alzheimer’s Risk
One of the lesser-discussed contributors to Alzheimer’s risk - particularly for APOE4 carriers - is ABCA1, a transport protein responsible for moving cholesterol out of cells and properly lipidating ApoE particles.
When ABCA1 function is suboptimal:
cholesterol lingers inside neurons and glial cells
membranes become stiffer
ApoE (already less efficient in APOE4) becomes poorly lipidated and more inflammatory
Importantly, this is rarely an “on/off” genetic defect. It’s usually a matter of efficiency.
ABCA1 activity appears to be supported by:
healthy membrane composition
adequate phospholipid availability
good mitochondrial function
regular physical activity
metabolic flexibility
lower chronic inflammation
In other words, cholesterol trafficking is not just a lab-number issue - it’s structural and functional.
Why Membrane Composition Matters So Much
Cell membranes aren’t passive barriers. They are working surfaces.
Their ability to move cholesterol depends on:
membrane fluidity
proper phospholipid balance
intact lipid raft structure
healthy mitochondrial and ER membranes
If membranes become:
rigid
oxidized
depleted of key phospholipids
then cholesterol export becomes inefficient even when transport proteins are present.
It’s like having delivery trucks waiting at a loading dock with no staff to unload. The system exists - but nothing moves smoothly.
Where APOE4 Complicates the Picture
APOE4 introduces several overlapping vulnerabilities:
1. Poor ApoE Lipidation
APOE4 forms less well-lipidated ApoE particles, meaning cholesterol has fewer high-quality carriers and lingers longer inside cells.
2. More Rigid, Cholesterol-Dense Membranes
APOE4 brains tend to show:
higher membrane cholesterol content
altered lipid raft structure
reduced membrane fluidity
Rigid membranes translate into sluggish transporter function.
3. Structural Stress on ABCA1
ABCA1 does not operate in isolation. It depends on:
adequate phosphatidylcholine
sufficient plasmalogen content
intact membrane curvature and flexibility
When membranes are phospholipid-poor or oxidatively damaged, ABCA1 efficiency drops - even if the gene itself is active.
Why This Matters Especially for the Brain
Neurons and glial cells:
synthesize their own cholesterol
rely heavily on local recycling and efflux
tolerate cholesterol accumulation poorly
When efflux falters:
synaptic membranes stiffen
receptor signaling degrades
inflammation rises
amyloid processing shifts in an unfavorable direction
This helps explain why APOE4 brains can show pathology even without markedly elevated blood cholesterol. It’s a cellular trafficking problem, not simply a serum lipid issue.
Where Phospholipids and Plasmalogens Fit In
Without overreaching beyond the data, the logic is straightforward:
Phosphatidylcholine provides structural material for ApoE and HDL particles
Plasmalogens support membrane fluidity and antioxidant defense
Healthier membranes improve transporter performance
Better transporter performance improves cholesterol efflux
Improved efflux reduces intracellular cholesterol stress in neurons
This doesn’t “fix” APOE4 - but it may well reduce one of its most important structural bottlenecks.
Where Dietary Fats Quietly Reinforce This Framework
Alongside targeted phospholipid support, I think it’s important not to overlook the quieter, foundational role of dietary fats that integrate into membranes over time. Extra virgin olive oil provides monounsaturated fats and polyphenols that support membrane fluidity while reducing oxidative stress within the lipid bilayer, particularly in neuronal and mitochondrial membranes. In parallel, supplemental omega-3s - with an emphasis on DHA - supply a fatty acid that is structurally essential for synaptic membranes, lipid rafts, and mitochondrial efficiency. DHA is not simply a signaling molecule; it is a literal building block of neuronal architecture. Taken consistently, these fats don’t replace Lipid Replacement Therapy, but they help ensure that when phospholipids and plasmalogens are supplied, they are incorporated into a membrane environment that is flexible, resilient, and less prone to inflammatory signaling.
Why Pulsing Lipid Support Makes Sense
Efflux systems don’t benefit from constant stimulation.
They benefit from repair, followed by time to function normally.
How I Personally Think About LRT (as APOE4/4)
I don’t use Lipid Replacement Therapy because I’m chasing perfection or trying to micromanage biology.
Because I’m APOE4/4, I know my brain depends heavily on healthy lipids — and I also know lipid handling isn’t always efficient or forgiving. My goal isn’t to push lipids constantly, but to ensure I’m not quietly missing important building blocks over time.
How I Use It, in Plain Terms
I use phosphatidylcholine as the base, specifically BodyBio PC, (*use code APOE4 for a discount) because it’s clean, gentle, and after using it for several years, I trust the brand!
I take it with meals, split across the day, without obsessing over dose. I’m simply supplying raw material during periods when I’m intentionally supporting membrane structure.
At times, I layer in plasmalogens, using Prodrome Glia and Prodrome Neuro. I think of these as more brain-specific lipids - especially relevant as plasmalogen levels decline with age and appear tied to Alzheimer’s risk.
I don’t take these continuously. I use / pulse them intentionally.
The Rhythm That Feels Right to Me
My pattern is simple:
several weeks on (PC plus plasmalogens)
several weeks off, with no lipid-specific supplementation
During the “on” phase, I support structure.
During the “off” phase, I let the system integrate and function.
That rhythm feels far more biologically appropriate than constant daily input.
One very simple, low-effort addition I rely on to support Lipid Replacement Therapy is eating small fatty fish - usually sardines or mackerel - about three times per week. I’ll be honest: sardines aren’t everyone’s favorite, so I often make them into patties, which makes them far more appealing without the fishy taste. I think of this as a practical safety net. Because DHA and EPA need to be incorporated into cell membranes over time, getting them regularly from whole food provides a buffer in case absorption from supplements is imperfect or inconsistent, especially in a genotype where lipid trafficking is already less efficient. Given how central membrane integrity is to mitochondrial function, lipid trafficking, and synaptic resilience, this is an easy, sustainable habit that quietly reinforces the goals of Lipid Replacement Therapy without requiring precision or perfection.
A note for sterol hyperabsorbers:
A subset of individuals - including some APOE4 carriers - are sterol hyperabsorbers, meaning they absorb plant sterols more efficiently through NPC1L1. In these cases, foods rich in phytosterols (such as nuts, seeds, and certain plant oils) can raise circulating plant sterols and potentially contribute to arterial or cellular sterol burden. This does not negate the importance of membrane integrity or lipid replacement, but it does mean that fat selection matters. For sterol hyperabsorbers, focusing on low-sterol fat sources while supporting phospholipids, DHA, and membrane structure can help align lipid replacement goals with individual absorption biology. There is one test I’m aware of that clearly identifies sterol absorption status: the Boston Heart Diagnostics Cholesterol Balance Test. It measures cholesterol production markers (desmosterol and lathosterol) alongside absorption markers (beta-sitosterol and campesterol). For many years I consumed large amounts of flaxseed, believing it was beneficial - only to later discover that I am a sterol hyperabsorber and need to avoid foods high in plant sterols. In that context, Ezetimibe (Zetia), which blocks intestinal sterol absorption, has been particularly helpful.
Where Statins Fit in a Lipid Replacement Framework
A brief note on statins is warranted here, because Lipid Replacement Therapy is often misunderstood as being “anti-statin.” That is not how I think about it. As I discussed in a previous postprimarily reduce circulating cholesterol synthesis and particle burden, while LRT focuses on restoring membrane composition, mitochondrial integrity, and lipid trafficking at the cellular level. These are not redundant targets. In an APOE4 context, lowering inflammatory lipid flux in the bloodstream and improving the quality and resilience of cellular membranes can be complementary strategies rather than competing ones. Linking the two helps move the conversation away from ideology and toward systems-level biology.
Final Thought
For me, Lipid Replacement Therapy is about respecting structure.
Healthy membranes don’t guarantee cognitive longevity - but without them, everything else has to work harder than it should. Pulsing phosphatidylcholine and plasmalogens feels like a reasonable, measured way to ensure my brain isn’t quietly short on the materials it depends on most.
Disclaimer
This content is for educational and informational purposes only and reflects my personal research, my interpretation of scientific literature, and my individual experience. It is not intended as medical advice, diagnosis, or treatment, and should not be used as a substitute for professional medical care.
Discussions of Lipid Replacement Therapy (LRT), phosphatidylcholine, plasmalogens, APOE genotype, cholesterol metabolism, ABCA1, or related mechanisms are theoretical and evolving. While grounded in current research, many concepts described are based on mechanistic reasoning, preclinical data, or limited human studies, and definitive clinical outcomes are not established.

Brilliant work linking membrane fluidity to ABCA1 function. The idea that cholesterol efflux isn't just about transporter expresion but about the membrane environment those transporters sit in is honestly something I needed to hear more clearly. I've been focused on optimizing omega-3s for months but never really thought through phosphatidylcholine as the literal foundation. The pulsing approach you describe makes way more sense than trying to supplement every single day.
Karen, great read! I have a few questions as I'm taking very similar Omega's as you and also eat salmon 2-3x/week.
Is there any data that going off/on with your Bio Body and Prodrome is better than continual? I've been doing it daily (along with a Nordic Naturals phospholipid Omega 3) but keep wondering if my blood is getting too thin.
I take 2 Bio Body phospholipid cholines and 2 Prodrome Neuro per day staggered along with the Nordic Naturals. Why do you take Glia Also?
Thanks so much and keep up the good work!!!